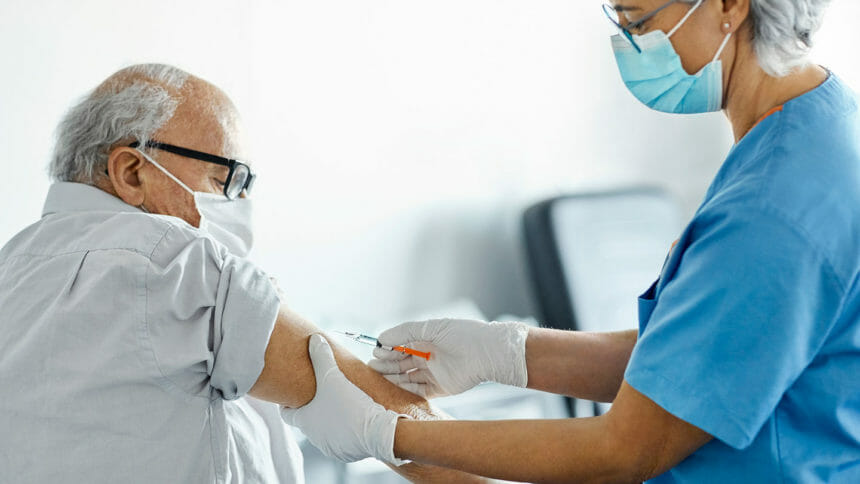
People aged 60 years and older may receive either of two recently approved vaccines for preventing respiratory syncytial virus (RSV) infections, vaccine advisers to the Centers for Disease Control and Prevention decided Wednesday. But the recommendation is not a sweeping endorsement.
In two separate votes for two older age groups, the panel concurred that clinical trial data supported use of the vaccines, made by Pfizer and GSK, based on shared decision-making between a patient and their physician.
The panel voted 9 to 5 in favor of vaccination for adults aged 65 and older. For patients aged 60 to 64, the panel voted 13 to 1 in favor of decisions made on an individual basis, reported the Center for Infectious Disease Research & Policy at the University of Minnesota.
Shared decision making, in this case, may also mean a patient-pharmacist discussion, as the vaccines are covered by Medicare Part D, and many will be administered in pharmacies rather than a doctor’s office, medical news outlet STAT reported.
The Food and Drug Administration in May approved GSK’s vaccine, Arexvy, and Pfizer’s vaccine, Abrysvo, specifically for older adults, who are highly vulnerable to severe infections. Arexvy’s formula includes an adjuvant, which can strengthen the immune response in patients with lowered immunity. Abrysvo does not contain an adjuvant. For each vaccine, clinical trials data show that only one dose may be needed to cover two years, with no additional benefit from another dose at 12 months, CIDRAP reported.
Fall season availability
An endorsement by the CDC’s director is the next step necessary before the vaccines can be released to the consumer market, and the companies are aiming to make the vaccines widely available in time for the fall season.
Like flu, RSV is highly contagious and causes seasonal outbreaks. Infections typically lead to mild symptoms, but can be deadly in vulnerable populations such as older and frail adults. In the United States, RSV leads to approximately 60,000 to 120,000 hospitalizations and up to 10,000 deaths each year among adults 65 years of age and older, according to the FDA.
The disease received more attention than usual during the fall of 2022, when the hospitalization rate for seniors was 10 times higher than usual for the season, according to a report by CNN at the time, citing federal data. The spike was attributed to the relaxation of pandemic mitigation measures, among other factors.
Notably, although RSV’s usual season is in winter, the virus has become more active in the late summer and early fall since the start of the pandemic, STAT reported.
A recent study by Mayo Clinic researchers and GSK found that cases of RSV in seniors are likely underestimated based on infection rates before and during the pandemic.
Related articles:
First-ever RSV vaccine to get FDA nod is approved for adults age 60+
FDA OKs second RSV vaccine option for older adults
Infectious disease experts call on clinicians to recognize threat of RSV in elders



