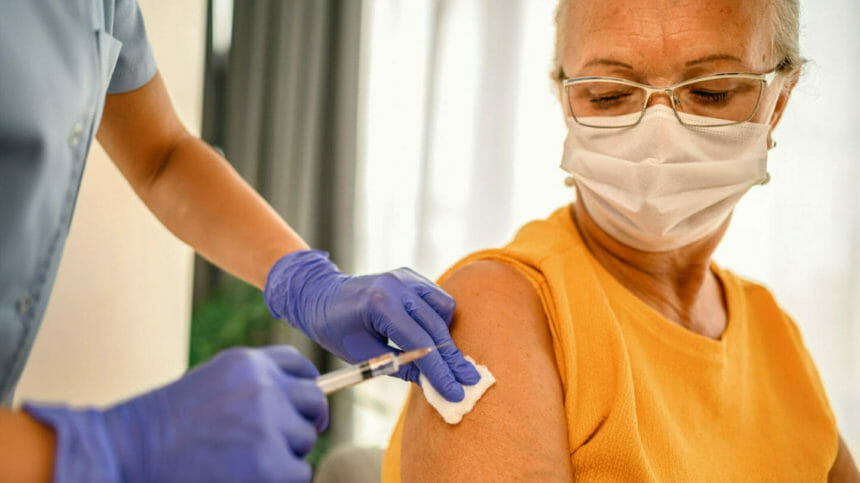
CDC director Rochelle Walensky has greenlit the use of two new respiratory syncytial virus (RSV) vaccines for preventing severe disease in older adults, emphasizing their use in vulnerable groups such as nursing home residents.
The agency announced the decision on Thursday, following a recommendation by its expert advisers, who recommended the vaccines be indicated for adults aged 60 years and older after consultation with their doctors. The shots, made by Pfizer and GSK, are the first to be licensed in the United States to protect against RSV, CDC said.
The sign-off is the last step on the path to commercial rollout following earlier approval by the Food and Drug Administration, and the vaccines are expected to become available in the fall.
RSV severely sickens up to 160,000 U.S. seniors each year. Some 10,000 older adults die annually from the infection, the CDC reported. The people most likely to experience severe RSV illness and hospitalization include older adults with chronic heart or lung disease and weakened immune systems and those living in nursing homes or long-term care facilities, it noted.
Anticipating fall virus season
“For the first time in U.S. history, people 60 years and older can now receive a vaccine for protection against the RSV virus,” Xavier Becerra, secretary of the Department of Health and Human Services (HHS), said in a statement following the CDC announcement. The decision to endorse the vaccines ensures that at-risk adults have strong protection against circulating respiratory viruses, he said.
“As we prepare for the fall vaccine campaign, we will follow the data and science to protect our nation’s most vulnerable older adults, those living in nursing or long-term care facilities, and the immunocompromised,” he said.
Related articles:
Infectious disease experts call on clinicians to recognize threat of RSV in elders
CDC advisers recommend RSV shot for older adults whose physicians are on board
First-ever RSV vaccine to get FDA nod is approved for adults age 60+



